✔ 100% Authentic Product
👁️ Currently Viewing 3020
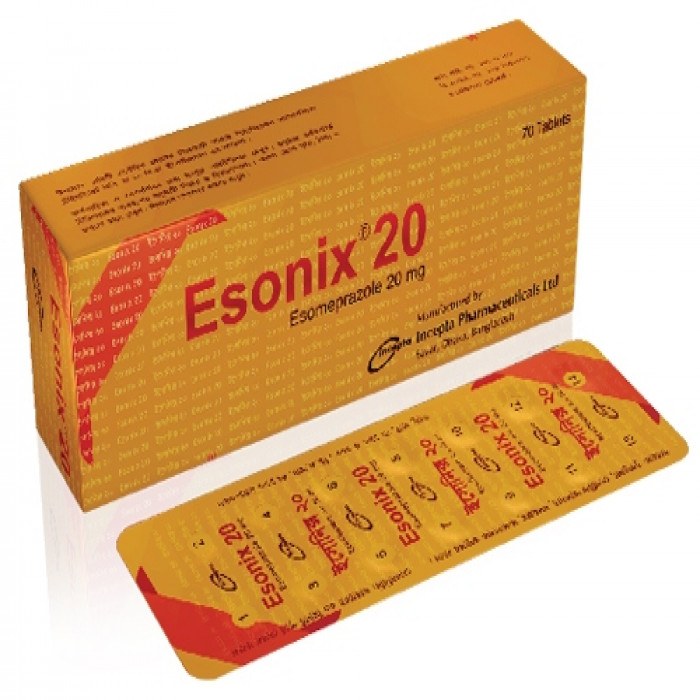
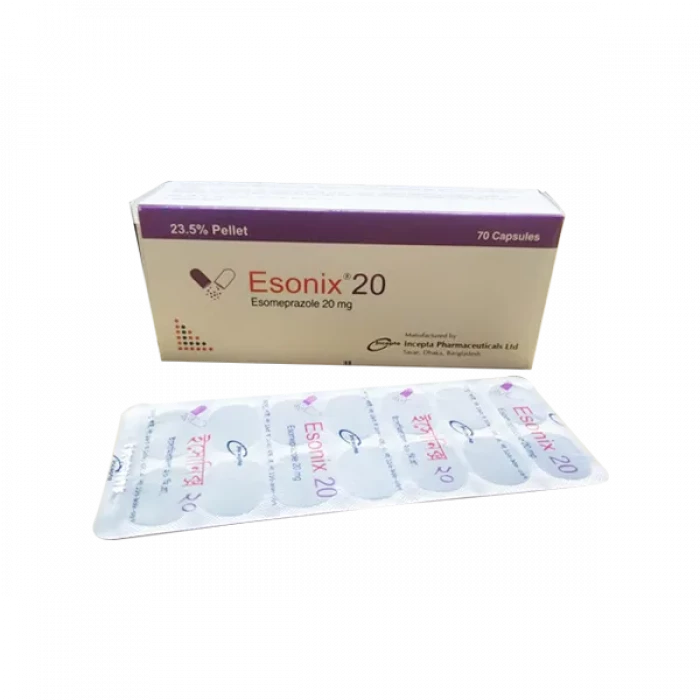
Esonix 20mg capsule 70Pcs (Box)
Capsule, Generic Name: Esomeprazole , Manufacturer: Incepta Pharmaceuticals Ltd.
Discount
Price: ৳ 526
MRP:
৳
560
6%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Indications of Esonix 20
Esomeprazole is indicated to relieve chronic heartburn symptoms and other symptoms associated with GERD.
Pharmacology of Esonix 20
Esomeprazole is a proton pump inhibitor that suppresses gastric acid secretion by specific inhibition of the H+/K+-ATPase in the gastric parietal cell. Esomeprazole (S-isomer of omeprazole) is the first single optical isomer of proton pump inhibitor, provides better acid control than racemic proton pump inhibitors.
Dosage
Healing of Erosive Esophagitis: 20 mg or 40 mg Once Daily for 4-8 Weeks. The majority of patients are healed within 4 to 8 weeks. For patients who don't heal after 4-8 weeks, an additional 4-8 weeks of treatment may be considered. Maintenance of Healing of Erosive
Esophagitis: 20 mg Once Daily (Clinical studies did not extend 6 months).
Symptomatic GERD: 20 mg Once Daily for 4 Weeks. If symptoms do not resolve completely after 4 weeks, an additional 4 weeks of treatment may be considered.
Helicobacter Pylori eradication: Triple Therapy to reduce the risk of Duodenal Ulcer recurrence-Esomeprazole 40 mg Once Daily for 10 days, Amoxicillin 1000 mg Twice Daily for 10 days, Clarithromycin 500 mg Twice Daily for 10 days.
Zollinger-Ellison Syndrome: The dose is 20-80 mg once daily. The dosage should be adjusted individually and treatment continued as long as clinically indicated.
Acid-related Dyspepsia: 20-40 mg once daily for 2-4 weeks according to the response.
Duodenal ulcer: 20 mg once daily for 2-4 weeks. Gastric ulcer: 20-40 mg once daily for 4-8 weeks.
Injection: The recommended adult dose is 40 mg Esomeprazole given once daily by intravenous injection (not less than 3 minutes) or intravenous infusion (10 to 30 minutes). Esomeprazole IV injection should not be administered concomitantly with any other medications through the same intravenous site. Treatment with Esomeprazole IV injection should be discontinued as soon as the patient is able to resume treatment with Esomeprazole delayed-release capsules. Safety and effectiveness in paediatric patients have not been established.
Administration
Esomeprazole tablet or capsule: should be swallowed whole and taken one hour before a meal.
Direction for use of Delayed-Release Oral Suspension: Whole contents of the packet should be taken into a small glass containing 15 ml. of water. The mixer should be stirred well and leave 2 to 3 minutes to thicken. Stir again and drink within 30 minutes. If any medicine remains after drinking, add more water, stir, and drink immediately. If the suspension is to be administered through a nasogastric or gastric tube, the volume of water in the syringe should be 15 ml. & immediately shake the syringe and leave 2 to 3 minutes to thicken. Shake the syringe and inject it through the nasogastric or gastric tube into the stomach within 30 minutes. An appropriately sized syringe should be used. Shake and flush any remaining contents from the nasogastric or gastric tube into the stomach.
Esomeprazole IV Injection: Esomeprazole IV should be given as a slow intravenous injection. The solution for IV injection is obtained by adding to the vial 5 ml of the solvent (WFI) provided. After reconstitution, the injection should be given slowly over a period of at least 3 minutes. The solution should be used within 12 hours of reconstitution when stored at room temperature up to 30°C. No refrigeration is required. The reconstituted solution should not be used if it contains visible particulate.
Interaction of Esonix 20
Esomeprazole is extensively metabolized in the liver by CYP2C19 and CYP3A4. In vitro and in vivo studies have shown that Esomeprazole is not likely to inhibit CYPs 1A2, 2A6, 2C9, 2D6, 2E1 and 3A4. No clinically relevant interactions with drugs metabolized by these CYP enzymes would be expected. Drug interaction studies have shown that Esomeprazole does not have any clinically significant interactions with phenytoin, warfarin, quinidine, clarithromycin or amoxicillin.
Contraindications
Esomeprazole is contraindicated in-patient with known hypersensitivity to any of the formulation.
Side Effects of Esonix 20
The most frequently occurring adverse events reported with Esomeprazole include headache, diarrhoea, nausea, flatulence, abdominal pain, constipation and dry mouth. There are no difference in types of related adverse events seen during maintenance treatment upto 12 months compared to short term treatment.
Pregnancy & Lactation
There are no adequate and well-controlled studies in pregnant women. Animal studies have revealed no teratogenic effects. The excretion of esomeprazole in milk has not been studied. Breast-feeding should be therefore be discontinued if the use of esomeprazole is considered essential.
Precautions & Warnings
General: Symptomatic response to therapy with esomeprazole does not preclude the presence of gastric malignancy.
Information for patients: Esomeprazole capsules should be taken at least one hour before meals. For patients who have difficulty swallowing capsules, one tablespoon of applesauce can be added to an empty bowl and the Esomeprazole capsules can be opened, and the pellets inside the capsule carefully emptied onto the applesauce. The pellets should be mixed with the applesauce and then swallowed immediately. The applesauce used should not be hot and should be soft enough to be swallowed without chewing. The pellets should not be chewed or crushed. The pellet/applesauce mixture should not be stored for future use. Antacids may be used while taking esomeprazole.
Therapeutic Class
Proton Pump Inhibitor
Storage Conditions
Store at a temperature not exceeding 30°C in a dry place. Protect from light and moisture. Keep out of reach of children.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.